Essential role of RelA Ser311 phosphorylation by zetaPKC in NF-kappaB transcriptional activation
- PMID: 12881425
- PMCID: PMC169043
- DOI: 10.1093/emboj/cdg370
Essential role of RelA Ser311 phosphorylation by zetaPKC in NF-kappaB transcriptional activation
Abstract
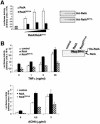
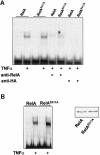
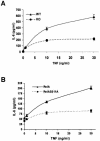
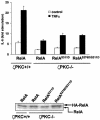
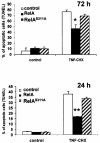
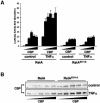
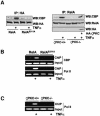
The activation of the transcription factor NF-kappaB is central to the control of the cellular response triggered by many stimuli. Once released from the inhibitory molecule IkappaB, NF-kappaB is translocated to the nucleus, and it has to be phosphorylated to activate transcription. In zeta protein kinase C (PKC)-deficient cells, NF-kappaB is transcriptionally inactive and the phosphorylation of the RelA subunit in response to tumor necrosis factor (TNF-alpha) is severely impaired. In vitro assays showed that zetaPKC directly phosphorylates RelA. Here we demonstrate that Ser311 accounts for zetaPKC phosphorylation of RelA and that this site is phosphorylated in vivo in response to TNF-alpha. Also, an inactivating mutation of that residue severely impairs RelA transcriptional activity, blocks its anti-apoptotic function and abrogates the interaction of RelA with the co-activator CBP as well as its recruitment, and that of RNA polymerase II (Pol II) with the interleukin-6 (IL-6) promoter. The interaction of endogenous CBP with endogenous RelA is inhibited in zetaPKC-/- cells, as well as the binding of Pol II to the IL-6 promoter. These results demonstrate the mechanism whereby zetaPKC regulates NF-kappaB activation in vivo.
Figures


Similar articles
-
Diacylglycerol kinase alpha enhances protein kinase Czeta-dependent phosphorylation at Ser311 of p65/RelA subunit of nuclear factor-kappaB.FEBS Lett. 2009 Oct 6;583(19):3265-8. doi: 10.1016/j.febslet.2009.09.017. Epub 2009 Sep 12. FEBS Lett. 2009. PMID: 19751727
-
RhoA mediates angiotensin II-induced phospho-Ser536 nuclear factor kappaB/RelA subunit exchange on the interleukin-6 promoter in VSMCs.Circ Res. 2006 Sep 29;99(7):723-30. doi: 10.1161/01.RES.0000244015.10655.3f. Epub 2006 Sep 7. Circ Res. 2006. PMID: 16960103
-
In vivo binding of NF-kappaB to the IkappaBbeta promoter is insufficient for transcriptional activation.Biochem J. 2006 Nov 15;400(1):115-25. doi: 10.1042/BJ20060786. Biochem J. 2006. PMID: 16792530 Free PMC article.
-
Regulation and function of IKK and IKK-related kinases.Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review.
-
Function for NF-kB in neuronal survival: regulation by atypical protein kinase C.J Neurosci Res. 1999 Dec 1;58(5):607-11. doi: 10.1002/(sici)1097-4547(19991201)58:5<607::aid-jnr1>3.0.co;2-m. J Neurosci Res. 1999. PMID: 10561688 Review.
Cited by
-
The new 4-O-methylhonokiol analog GS12021 inhibits inflammation and macrophage chemotaxis: role of AMP-activated protein kinase α activation.PLoS One. 2015 Feb 23;10(2):e0117120. doi: 10.1371/journal.pone.0117120. eCollection 2015. PLoS One. 2015. PMID: 25706552 Free PMC article.
-
Polarity protein alterations in carcinoma: a focus on emerging roles for polarity regulators.Curr Opin Genet Dev. 2010 Feb;20(1):41-50. doi: 10.1016/j.gde.2009.12.001. Epub 2010 Jan 21. Curr Opin Genet Dev. 2010. PMID: 20093003 Free PMC article. Review.
-
Activin upregulation by NF-κB is required to maintain mesenchymal features of cancer stem-like cells in non-small cell lung cancer.Cancer Res. 2015 Jan 15;75(2):426-35. doi: 10.1158/0008-5472.CAN-13-2702. Epub 2014 Nov 28. Cancer Res. 2015. PMID: 25432175 Free PMC article.
-
Nephrin deficiency activates NF-kappaB and promotes glomerular injury.J Am Soc Nephrol. 2009 Aug;20(8):1733-43. doi: 10.1681/ASN.2008111219. Epub 2009 Jun 4. J Am Soc Nephrol. 2009. PMID: 19497968 Free PMC article.
-
PTEN Is Required for The Anti-Epileptic Effects of AMPA Receptor Antagonists in Chronic Epileptic Rats.Int J Mol Sci. 2020 Aug 6;21(16):5643. doi: 10.3390/ijms21165643. Int J Mol Sci. 2020. PMID: 32781725 Free PMC article.
References
-
- Anrather J., Csizmadia,V., Soares,M.P. and Winkler,H. (1999) Regulation of NF-κB RelA phosphorylation and transcriptional activity by p21(ras) and protein kinase Cζ in primary endothelial cells. J. Biol. Chem., 274, 13594–13603. - PubMed
-
- Claudio E., Brown,K., Park,S., Wang,H. and Siebenlist,U. (2002) BAFF-induced NEMO-independent processing of NF-κB2 in maturing B cells. Nat. Immunol., 3, 958–965. - PubMed
-
- Cowley S., Paterson,H., Kemp,P. and Marshall,C.J. (1994) Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell, 77, 841–852. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

